Stratified Medicine Paediatrics (SMPEDS)

Program overview
A UK wide initiative, CRUK-Stratified Medicine Paediatrics (StratMedPaediatrics) began in the UK in 2019 as a national prospective multi-omic sequencing and biomarker reporting platform across 20 Paediatric Trusts. Whilst the original StratMedPaeds1 study is now closed to recruitment, a new research focussed StratMedPaeds 2 study has recently begun, with opening and first recruitment planned from August 2024.
The original study, StratMedPaeds1 (2018-2023) established a national pathway for biomarker validation in tissue and blood in paediatric solid tumours (and more recently leukaemia). StratMedPaediatrics 1 sequenced >700 patients, and validated three genomic tests (DNA panels, RNA-fusion panels, low-copy WGS) for use within the NHS. A major outcome of the programme was the adoption of both DNA panel and RNA-fusion panel sequencing, in addition to NHS whole genome sequencing (short-read WGS), through GMSA hubs for children with cancer into NHS SoC. Additionally, StratMedPaediatrics, publishing the technical parameters of plasma ctDNA panel testing in a large cohort of paediatric plasma samples, in support of our application to the NHS to have liquid biopsy accredited as a clinically valid test methodology in 2021. In the latter stages of StratMedPaediatrics 1, we have continued to focus on data analysis and dissemination, and on the development of next-generation diagnostic technologies that could deliver more information from less tissue or ideally from blood.
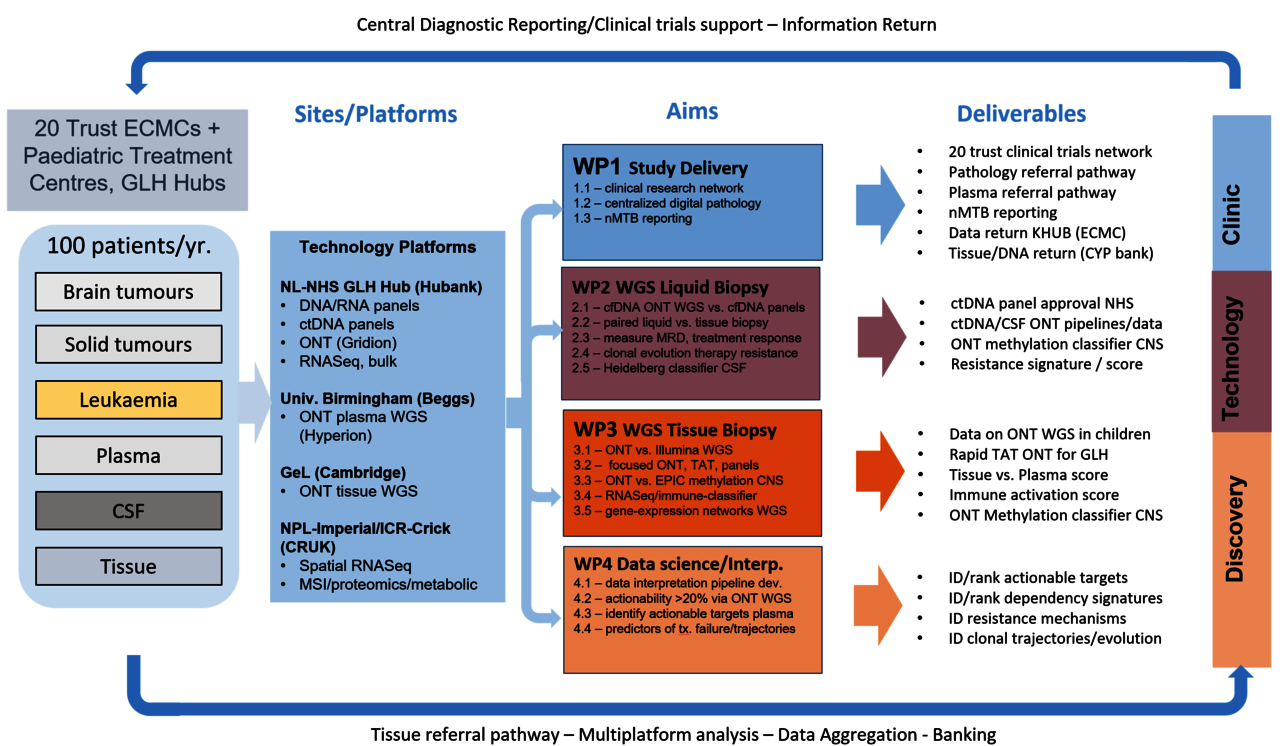
StratMedPaediatrics 2 (2023-) will assess whether new platforms, and liquid biopsy in particular, can: 1) identify more patients for treatment on biomarker-stratified experimental clinical trials, 2) be used serially to monitor treatment response, and 3) detect emergence of treatment-resistant disease. StratMedPaediatrics 2 used cutting-edge research sequencing platforms that operate in the <50ng ctDNA input range and with rapid turnaround, in plasma. We will focus on rapid turnaround (TAT) in tissue (ie: <1 week, target 48-72h) and will operate in partnership with molecularly enriched and adaptive national and international clinical trials inform on their conduct. As such StratMedPaediatrics 2 is a key element within our national strategy and vision to provide the technical validation, serial monitoring infrastructure and reporting structures to facilitate a new era of biomarker-guided trials, which we hope will translate into the routine implementation of real-time monitoring of their disease and response to therapy.
StratMedPaediatrics 2 sits alongside NHS SoC testing, using our established sample processing, analysis, and reporting pathways, to validate advanced molecular techniques in both tumour tissue and blood, to accelerate our ability to rapidly identify, and serially monitor molecular biomarkers in support of advanced clinical trials of biologically-targeted novel therapies. The study has 4 aims: 1) to expand a national infrastructure for research diagnostics, 2) to validate liquid biopsy as a non-invasive clinical diagnostic test, 3) to develop advanced tissue-based diagnostics that are vastly more informative, and 4) to develop novel bioinformatic approaches that will identify treatable events in a majority of children who relapse.
In partnership with CRUK And Genomics England, StratifiedMedicinePaediatrics2 pilots the use of novel diagnostics in a large cohort of childhood cancer patients, to identify and report on new biomarkers from biopsy-derived tumour tissue (using nanopore, spatial RNA sequencing and proteomics, mass spectroscopy imaging and immunoprofiling), blood and CSF. These new technologies will be assessed for their ability to deliver superior diagnostic accuracy more quickly and non-invasively than current NHS SoC.
The technical advances delivered here aim to deliver a step-change in the ability of clinicians to discern treatment targets, monitor the efficacy of treatment in blood, dynamically alter therapy and predict resistance in children with relapsing tumours. All of the data generated will be shared with an emerging richly-annotated clinical database on molecular alterations that characterise UK patients for future treatment.
Program details
StratMedPaediatrics 2 (as with its predecessor StratMedPaeds1) is a UK wide national study, sponsored by the Institute of Cancer Research and governed via full national UK ethical approval and run nationally through the Cancer Research UK Clinical Trials Unit, Birmingham. It is a prospective, multicentre biomarker evaluation study,
Patient Population: Eligible patients in the UK include children with relapsed or treatment refractory intracranial, extracranial solid tumours, or haematological malignancies (including
leukaemia, lymphoma and Langerhans Cell Histiocytosis) recruited from UK paediatric primary treatment centres. These represent >400 patients/yr. For study, this number is limited to a subset of patients for whom tissue-biopsy (mandatory for study entry, could be safely obtained within 8 weeks of study registration).
Inclusion and Exclusion Criteria
INCLUSION
- Patients with relapsed/refractory paediatric tumour (solid tumours, central nervous system (CNS) tumours, Leukaemia and Lymphoma)
- Formalin fixed paraffin embedded (FFPE) tumour available from a biopsy, resection or other surgical procedure or a viable bone marrow sample that was taken within 8 weeks of trial entry * (this requirement may be amended as liquid biopsy is validated, allowing replacement of invasive biopsy)
- Written informed consent of patient/parent/guardian
- No upper age limit, but eligibility mandates that the primary cancer is classified as a “paediatric-type malignancy”.
EXCLUSION
- Patients without a relapsed or refractory paediatric tumour (all solid tumours, central nervous system (CNS) tumours, Leukaemia and Lymphoma)
- Patients without a Formalin fixed paraffin embedded (FFPE) tumour sample or viable bone
marrow sample taken within 8 weeks of trial entry (note this requirement may be amended as liquid biopsy is validated, allowing replacement of invasive biopsy).
Benefits and outcomes
Expected clinical impact. StratMedPaediatrics and other national paediatric molecular profiling initiatives, identify pathogenic events in ~70% of cases, with a third leading to a treatment recommendation (clinical trial or specific targeted therapy), and 20-25% leading to a direct change in treatment. Germline cancer predisposition is found in approx. 8% of cases and diagnosis is altered or refined in 8-13%. The genetic events we detected in StratMedPaediatrics 1 using childhood cancer molecular platforms are now incorporated within standard (NHS funded) diagnostic sequencing and this has resulted in clinically significant information being available to local treating teams, patients and parents which can have a direct impact on the patient’s management. However, it remains the case that a majority of patients will not have a treatment recommendation made using current NHS diagnostics. Furthermore, WGS requires a relatively large amount of frozen tissue from an invasive biopsy and has to be performed on a serial basis. StratMedPaediatrics2 will pilot, validate, and then act as an infrastructure to deliver new biomarkers from both biopsy-derived tumour tissue (nanopore, spatial RNASeq, spatial mass spectroscopy imaging and immunoprofiling) and liquid serial samples (plasma and CSF). These new biomarkers aim to deliver equivalent or better results using less tissue, in a less invasive and quicker turnaround time to current SoC at a comparable or cheaper cost. Liquid serial samples will not only potentially be able to replace invasive biopsies27 but could also be used to detect and/or monitor early disease relapse or progression and provide an overall molecular analysis of all tumour subclones and metastatic lesions rather than one or two lesions via a traditional needle biopsy, which do not capture the genetic heterogeneity of human tumours.
Benefit to patients - outcome.
- Novel biomarkers stratify patients to therapy above current SoC.
- Incorporation of novel biomarkers into new clinical trials to improve design and efficiency.
- Potential to increase detected actionable events for targeted therapy or immunotherapy.
- Early detection of cancer relapse in blood prior to clinical evidence of relapse.
- Identification of treatment resistance mechanisms with potential to rapidly adapt therapy.
Benefit to patients - experience. StratMedPaediatrics2 will function to allow NHS funded standard of care molecular analysis to be combined and integrated with new biomarker research to initially allow optimisation and validation but subsequently allow reporting via the nMTB, as we have successfully performed in StratMedPaediatrics 1. This will include serial analysis of liquid samples (plasma and CSF) collected for longitudinal monitoring.
Specific patient benefits. successful identification and technical validation of rapid turnaround (<=14 days) biomarkers will provide new tools to select and monitor patients in clinical trials. The results of these trials based on the StratMedPaediatrics2 outcomes and infrastructure, are anticipated to lead to incorporation into SoC and improve patient experience through refinement of diagnosis, identification of targetable tumour biology allowing entry onto experimental clinical trials, and early detection of recurrence or new cancers potentially speeding up diagnosis and treatment stratification in the relapse setting. As plasma sequencing becomes validated, we anticipate that it will be included in the NHS test directory during the lifetime of this programme and would change the way in which patients and clinicians regard liquid sample-based sequencing results. We hope that CSF monitoring will provide the same potential for patients with central nervous system tumours
Expert team
Louis Chesler, Chief Investigator and Study Lead, Institute of Cancer Research
Darren Hargrave, Co-study lead: Clinical Delivery, Great Ormond Street Hospital
Isidro Cortes Ciriano, co-study lead: Data, EBI/EMBLEM
Maggie Cheang, WP Lead: Biostatistics and Spatial analysis, Institute of Cancer Research
Amos Burke, WP Lead: Clinical Trial Operations, Director, CRCTU, Birmingham
Mike Hubank, WP Lead: Molecular Genetics, Institute of Cancer Research
Tom Jacques, WP Lead: Pathology, UCL/Great Ormond Street
Ciaran Hutchinson, WP Lead: Pathology, UCL/Great Ormond Street
Andrew Beggs, WP lead: Nanopore Sequencing, Birmingham University
John Anderson, WP Lead: Immunology, UCL/Great Ormond Street
Chris Bakal, WP lead: Mathematical Modelling/AI, Institute of Cancer Research
Steve Clifford, CNS and Brain clinical trials, Newcastle University
Tim Ritzmann, CNS and Brain clinical trials, University of Nottingham
Sally Halls, PPIE, Blue Skye Thinking
John Rainsbury, PPIE, Little Hero Brain Tumour Charity
Technology and Innovation